HOST INSTITUTION
Centre national de la recherche scientifique (CNRS)/ Université de Bordeaux
EMAIL ADDRESS
lucio.di-nicola@u-bordeaux.fr
SUPERVISOR
Dr. Yann Ferrand
BRIEF CV
Born in 1999 in Italy, Lucio obtained a Bachelor’s Degree in Chemistry with honors (cum laude) from the University of Parma in July 2021. He worked in the research group of Professors Francesco Sansone and Alessandro Casnati, defending his thesis titled “Synthesis and Characterization of New Lipophilic Analogues of Tolcapone as Stabilizers of the Transthyretin Tetramer for the Treatment of Amyloidosis”. In 2024, he earned a Master’s Degree in Organic Chemistry from the University of Pisa with a grade of 110/110. During his Master’s internship, he worked in the laboratories of Professors Gaetano Angelici and Gennaro Pescitelli on the synthesis of chiral peptoids, completing a thesis entitled “Synthesis, Characterization, and Metal-Binding Studies of Chiral Peptoid-Based Chelators.” In October 2024, he was hired by the Marie Skłodowska-Curie Actions Doctoral Networks and began his PhD within the ENSCC (European Network on the Supramolecular Chemistry of Carbohydrates), under the supervision of Dr. Yann Ferrand (BISE Research Group, IECB, Bordeaux, France). Currently, he is working on the synthesis of aromatic oligoamide foldamers for the selective recognition of sugars and as supramolecular protecting groups for selective chemistry of carbohydrates in organic solvents.
PROJECT DESCRIPTION
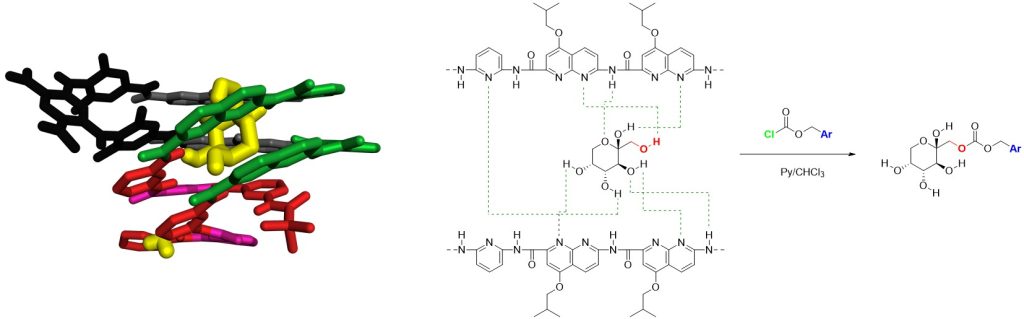
Through this thesis project, we aim to draw inspiration from the recognition process carried out by proteins by utilizing a class of synthetic molecules known for their predictable folding behavior: aromatic oligoamide foldamers. These molecules are designed to fold into specific shapes, such as helices, with diameters that can be tuned to create cavities with particular geometric properties. Indeed, by adjusting the type of monomers employed it is possible to generate molecular containers such as capsules or cones. These cavities are lined with multiple hydrogen bond donors and acceptors and can effectively interact with and encapsulate guest molecules, including carbohydrates, through non-covalent interactions.
The ability of these foldamers to recognize and bind specific molecules makes them promising candidates for use in supramolecular catalysis, where they can facilitate chemical reactions in a highly selective manner, similar to the way enzymes function. Furthermore, their structural versatility allows them to serve as supramolecular protecting groups, capable of shielding multiple reactive sites simultaneously, thus enabling the selective functionalization of only certain functional groups. In both contexts, the foldamers mimic the first crucial step of enzymatic activity: molecular recognition.
MAIN RESEARCH FIELD
Organic Chemistry, Supramolecular Chemistry